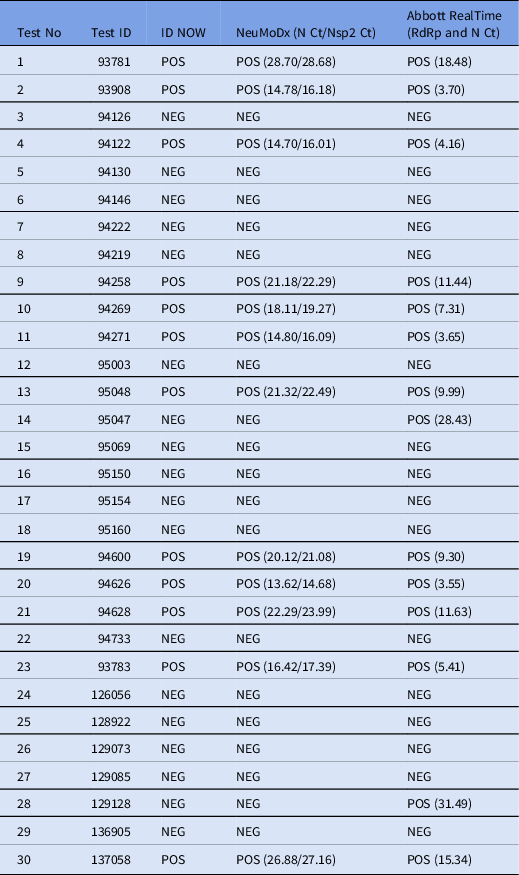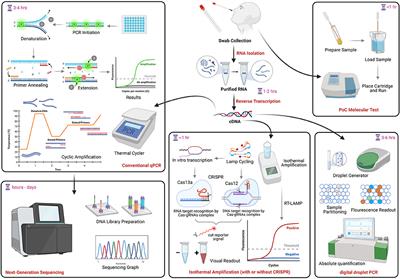
Diagnostics | Free Full-Text | COVID-19 Infection Diagnosis: Potential Impact of Isothermal Amplification Technology to Reduce Community Transmission of SARS-CoV-2
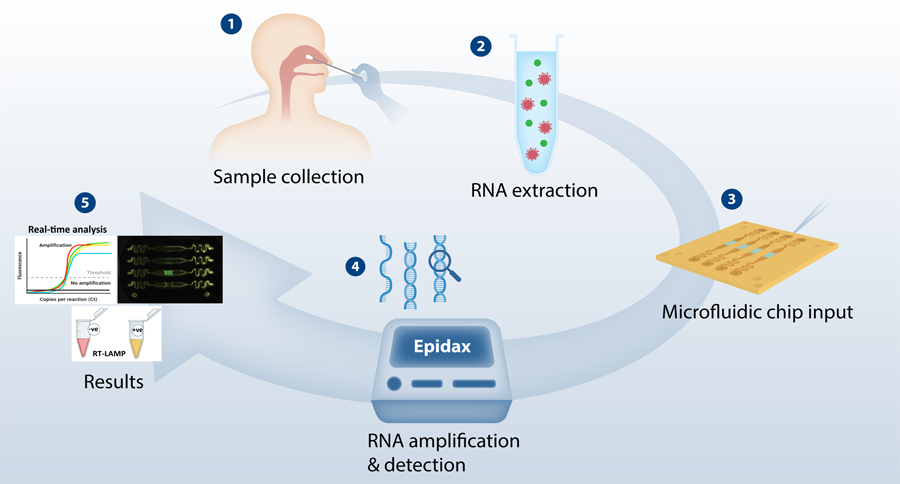
Modular micro-PCR system for the onsite rapid diagnosis of COVID-19 | Microsystems & Nanoengineering
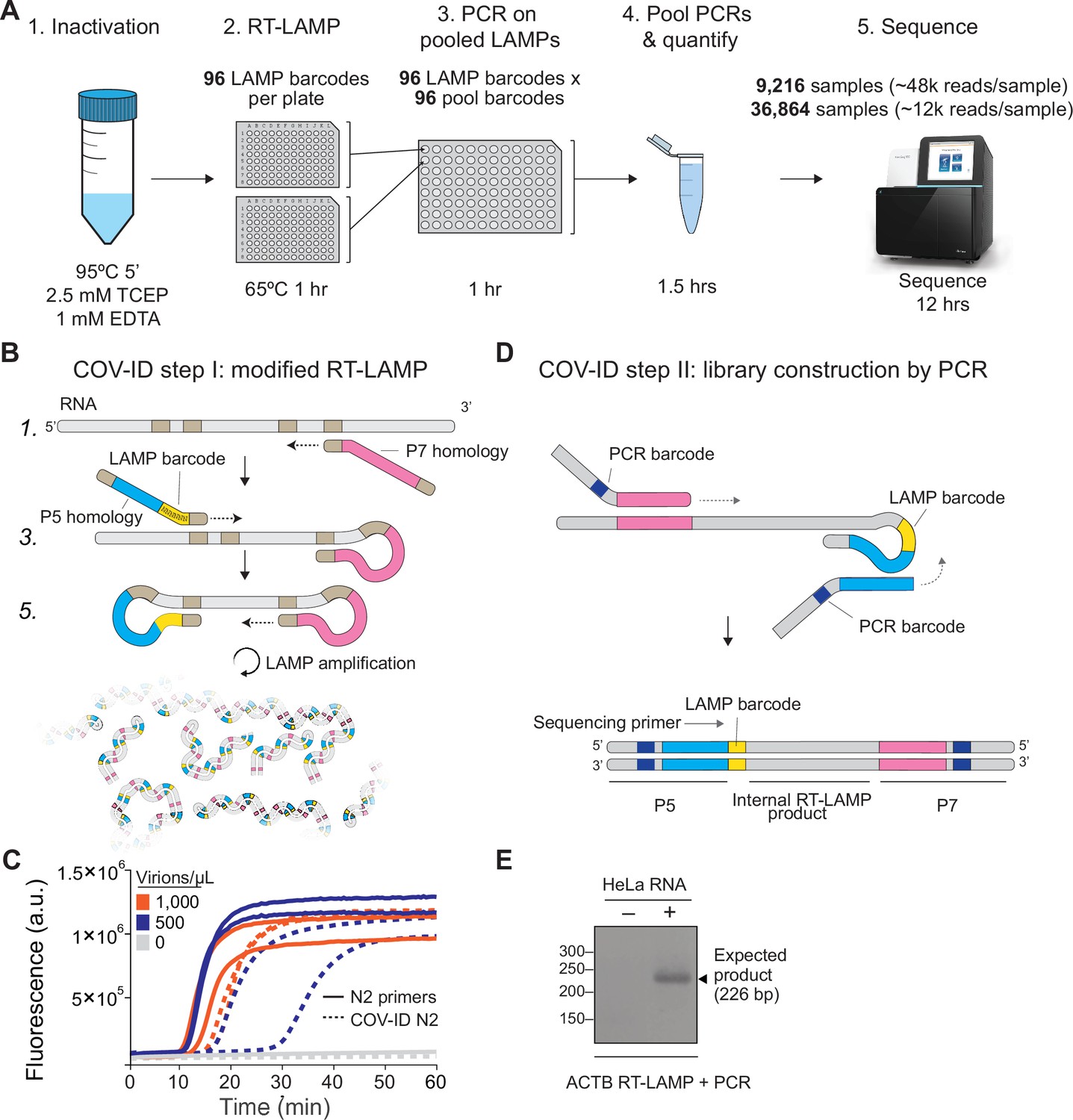
A LAMP sequencing approach for high-throughput co-detection of SARS-CoV-2 and influenza virus in human saliva | eLife
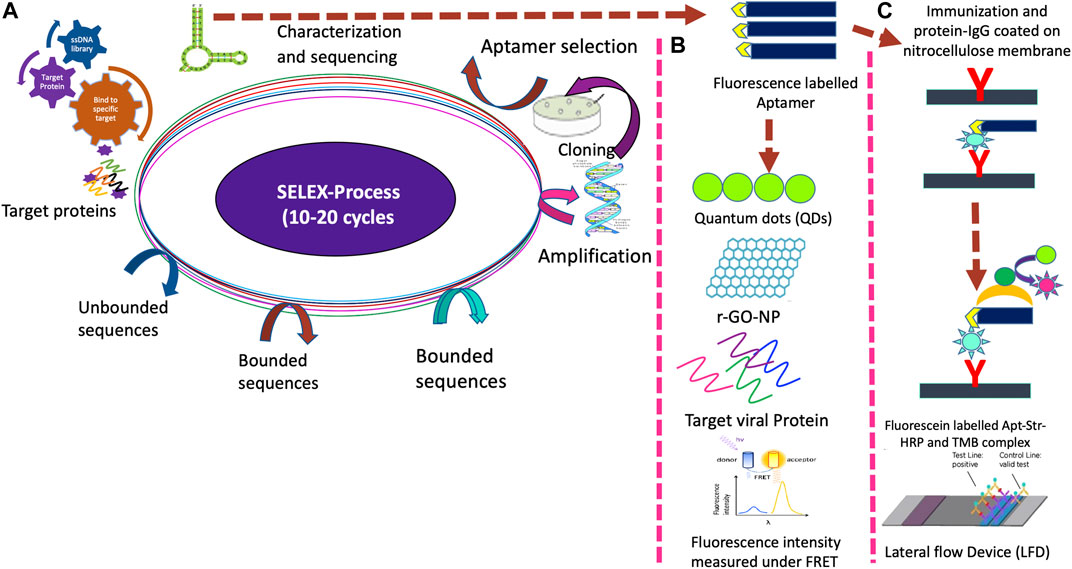
Frontiers | Point of Care Diagnostic Devices for Rapid Detection of Novel Coronavirus (SARS-nCoV19) Pandemic: A Review

Sensitivity and potential utility of SARS-CoV-2 rapid antigen and nucleic acid amplification tests in the context of an elimination approach | OPEN ACCESS

Abbott Launches Molecular Point-of-Care Test to Detect Novel Coronavirus in as Little as Five Minutes - Mar 27, 2020

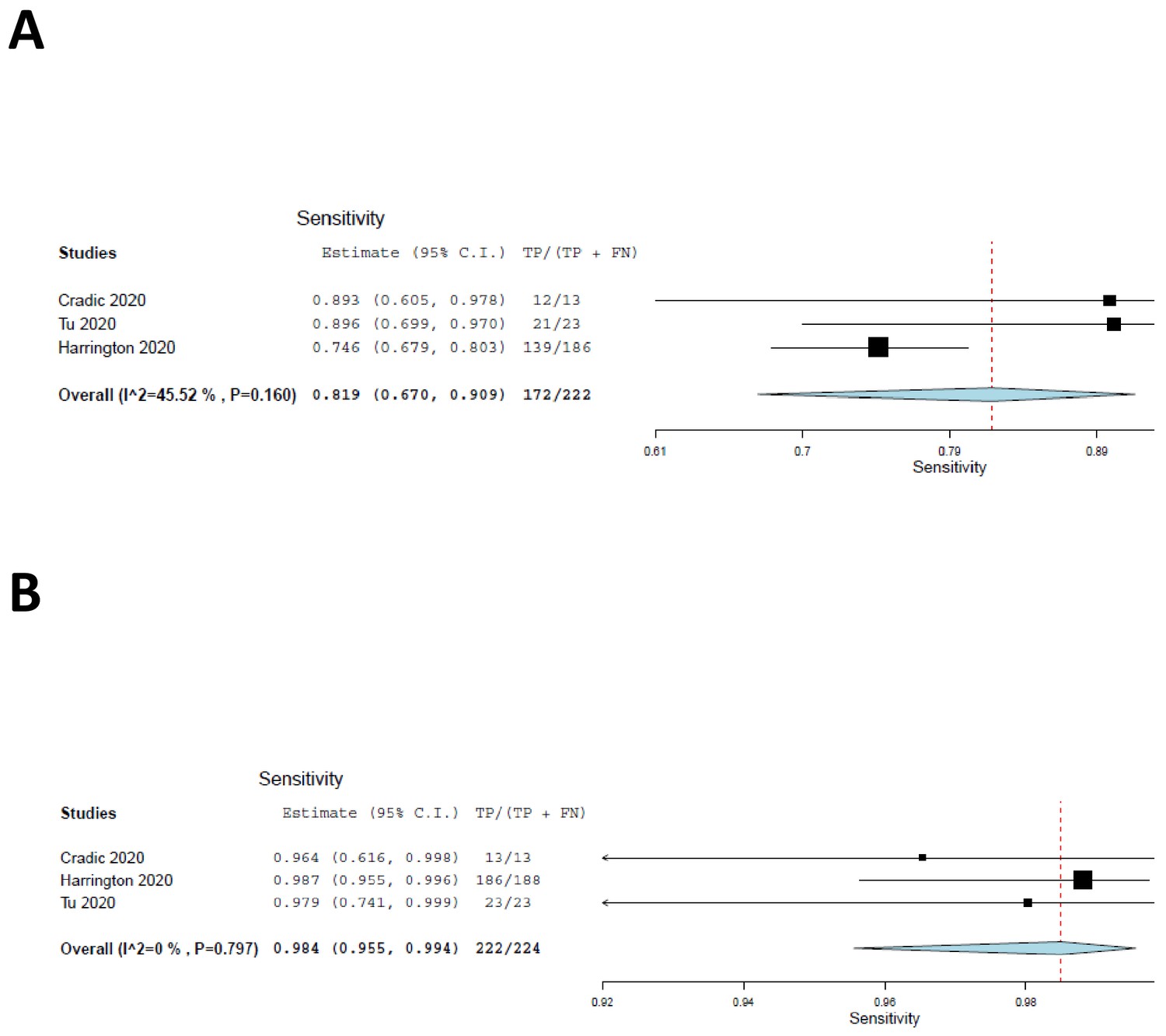
The diagnostic accuracy of isothermal nucleic acid point-of-care tests for human coronaviruses: A systematic review and meta-analysis | Scientific Reports
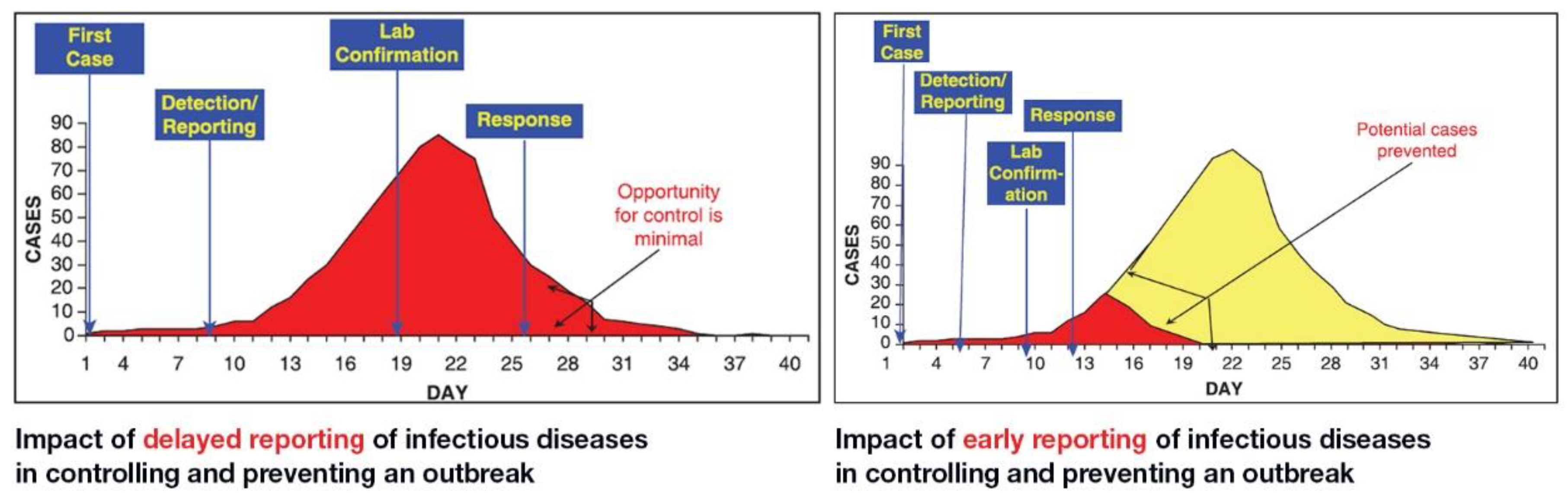
Diagnostics | Free Full-Text | COVID-19 Infection Diagnosis: Potential Impact of Isothermal Amplification Technology to Reduce Community Transmission of SARS-CoV-2

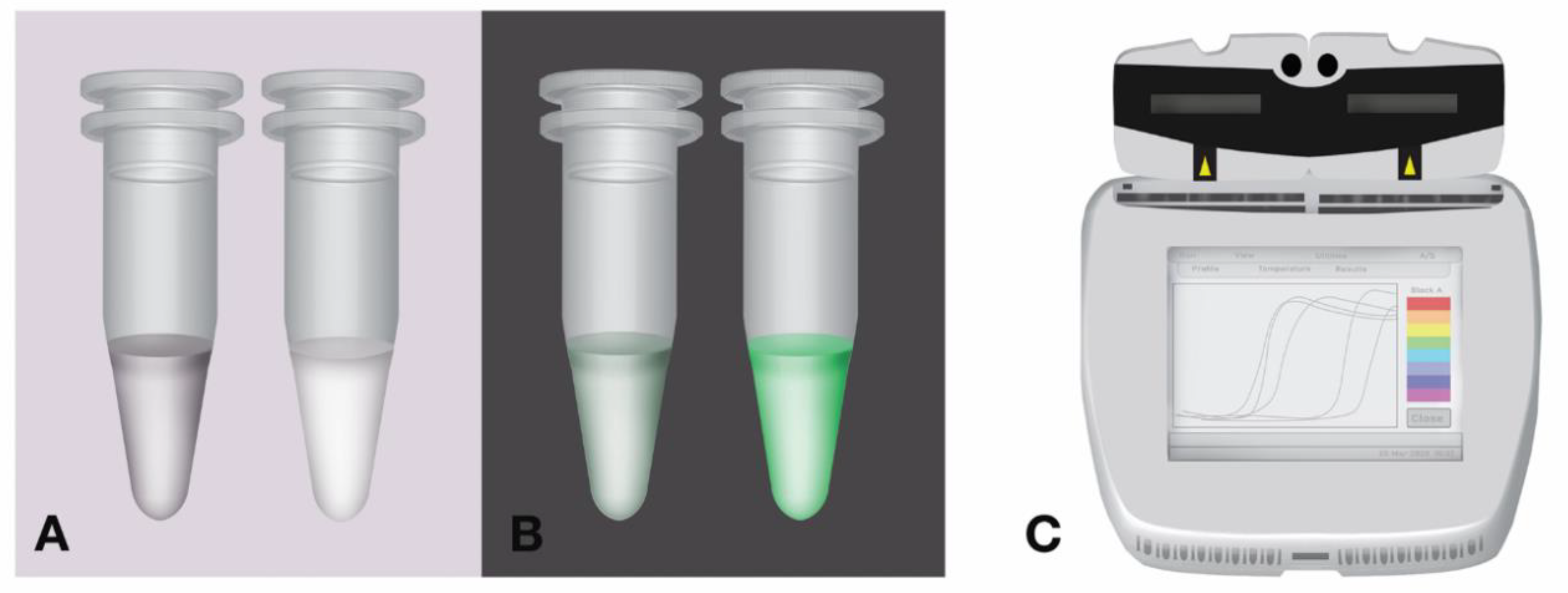
Diagnostic performance of a colorimetric RT -LAMP for the identification of SARS-CoV-2: A multicenter prospective clinical evaluation in sub-Saharan Africa - eClinicalMedicine