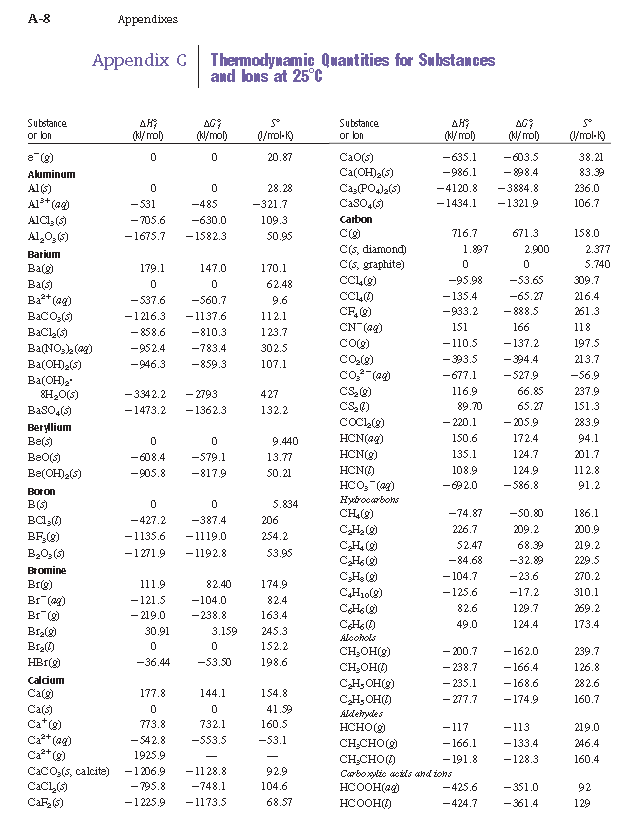
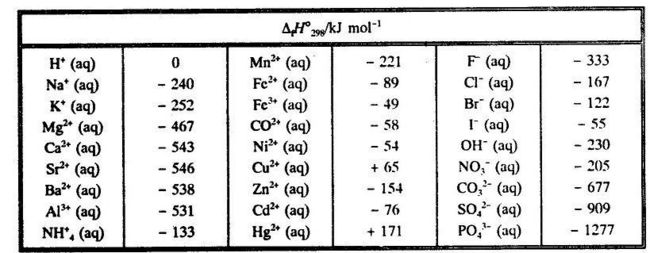
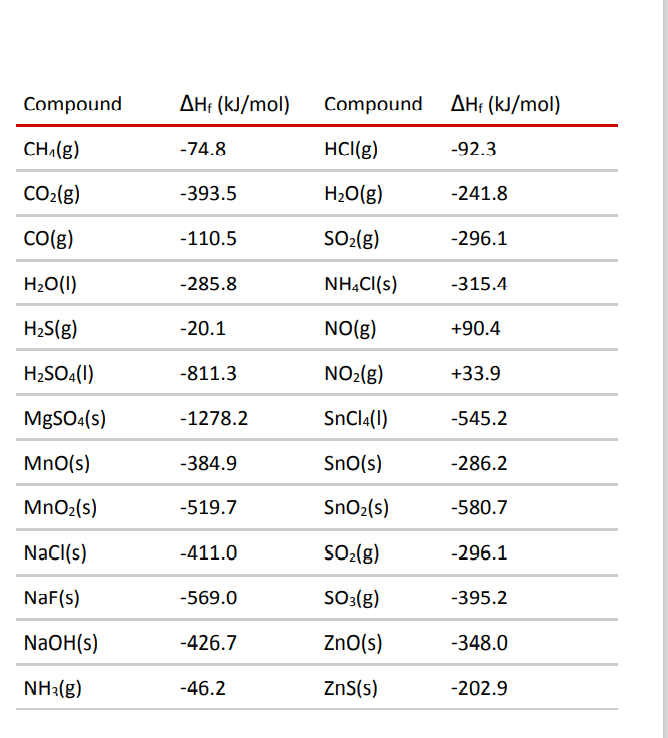
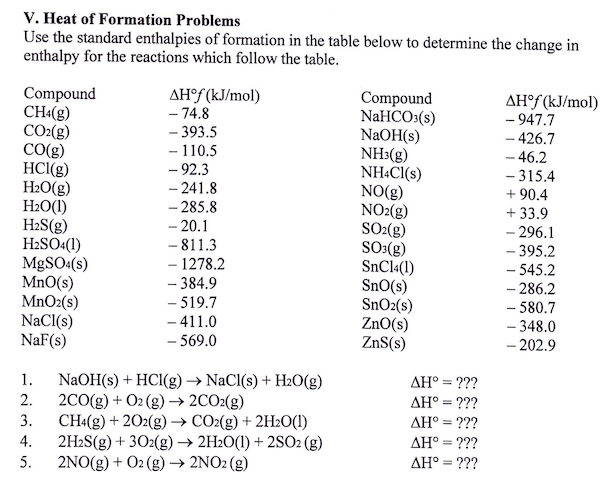
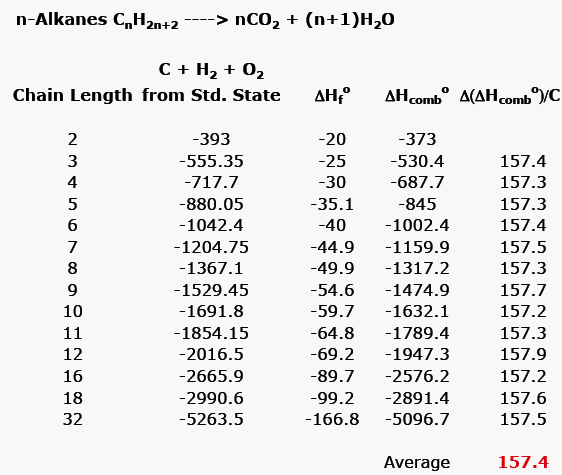
Table 6 from Efficient Estimation of Formation Enthalpies for Closed-Shell Organic Compounds with Local Coupled-Cluster Methods. | Semantic Scholar
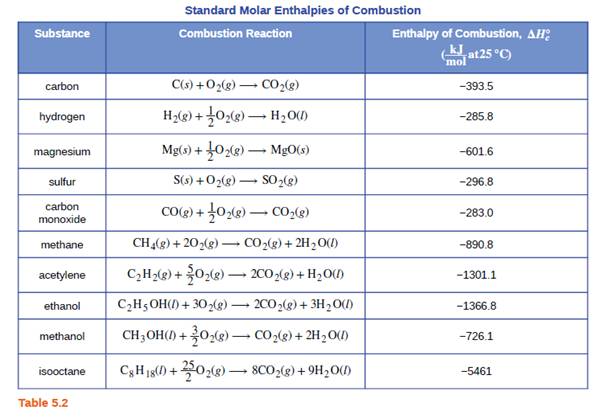
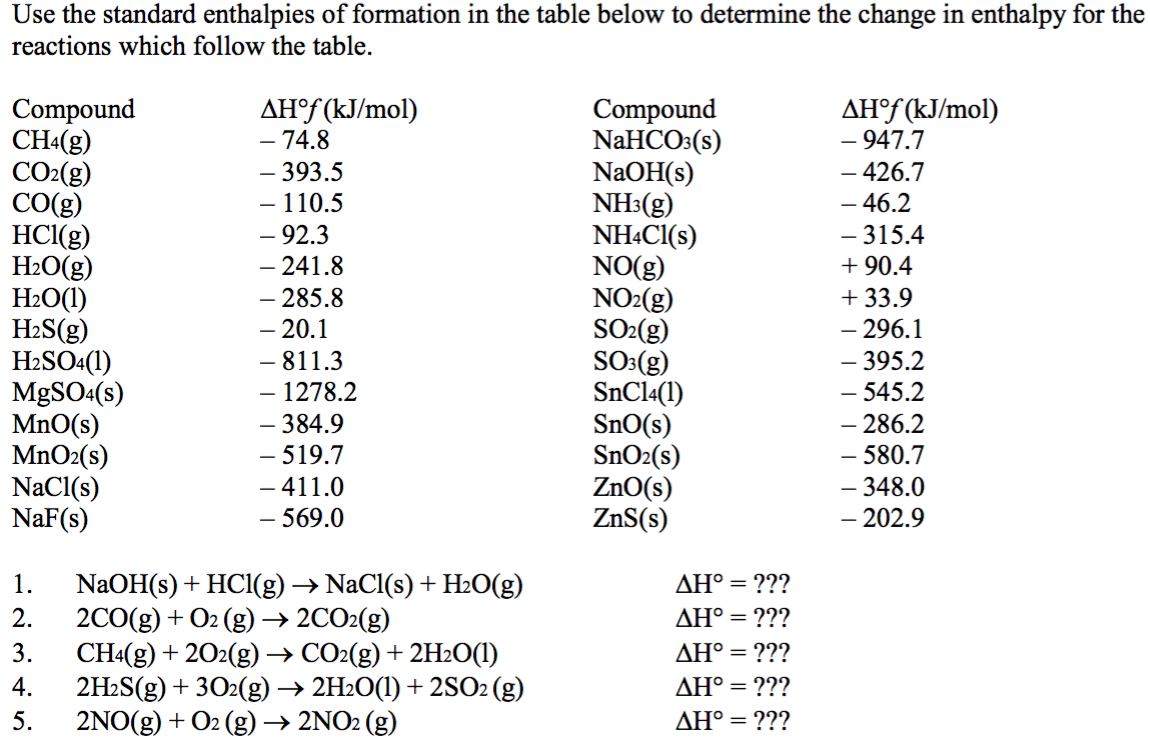
Which of the enthalpies of combustion in Table 5.2 the table are also standard enthalpies of formation? | bartleby

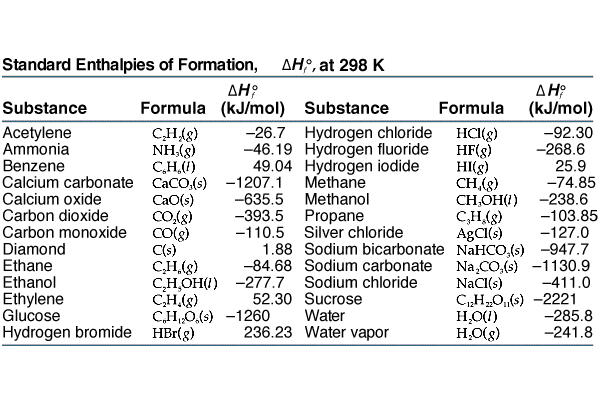
STANDARD HEAT OF FORMATION. DEFINITION The change in enthalpy that accompanies the formation of one mole of the compound at its normal state from its. - ppt download

Table 3 from Group additivity values for enthalpies of formation (298 K), entropies (298 K), and molar heat capacities (300 K < T < 1500 K) of gaseous fluorocarbons | Semantic Scholar

SciELO - Brasil - Estimation of standard enthalpy of formation of alkanes in gaseous state by calculating size, structural and electronic parameters in the molecules Estimation of standard enthalpy of formation of
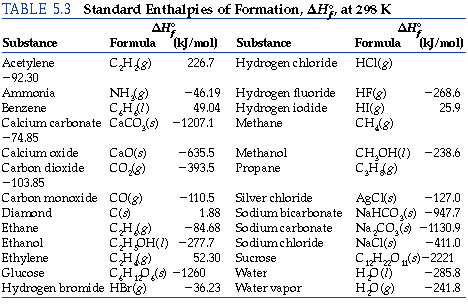
Calculate the enthalpy of reaction for the reaction "CH"_3"COOH" + "H"_2"O" -> "CH"_3"CH"_2"OH" + "O"_2? | Socratic
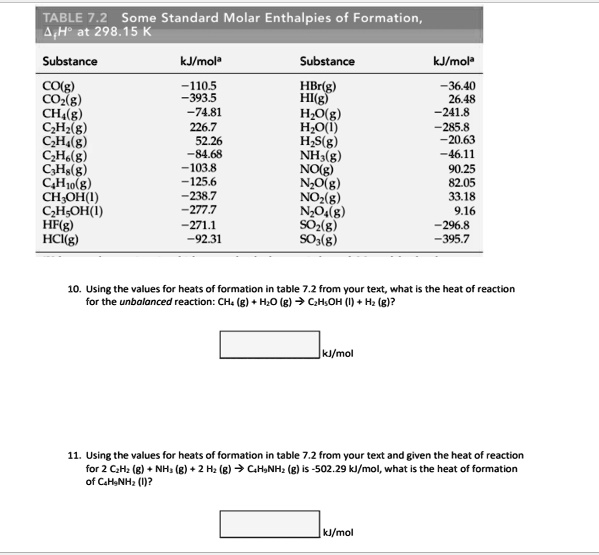

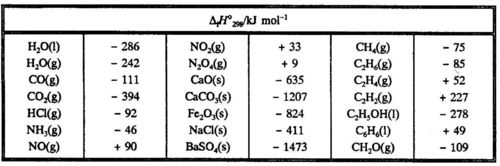
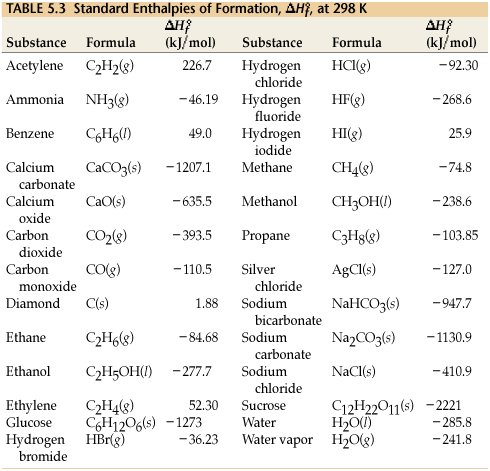
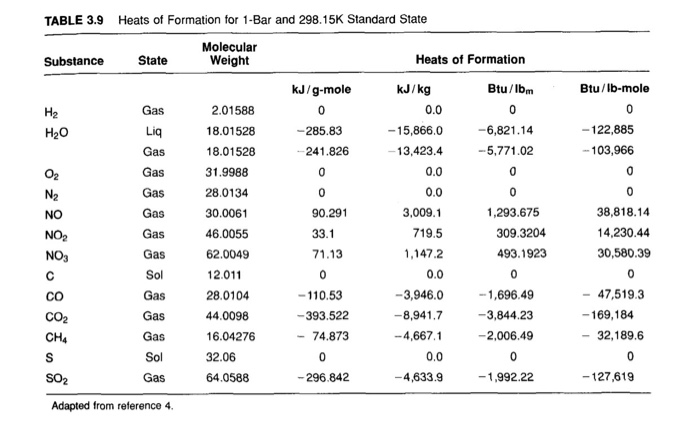
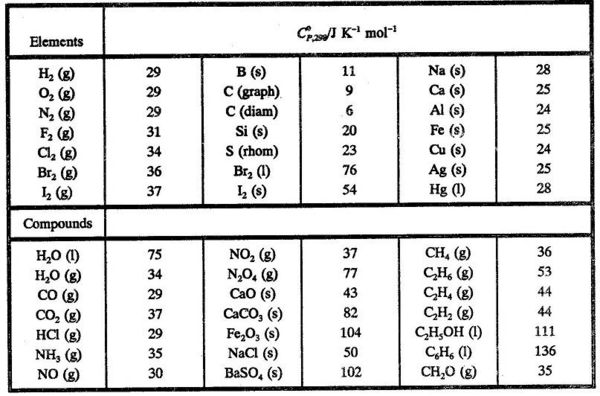
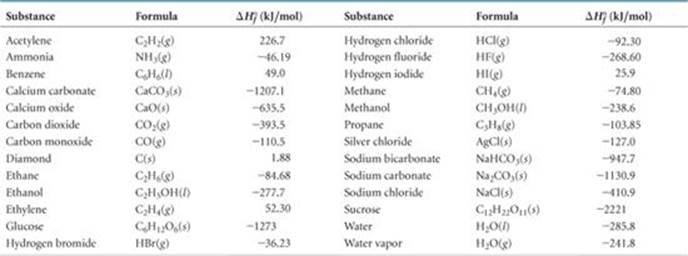
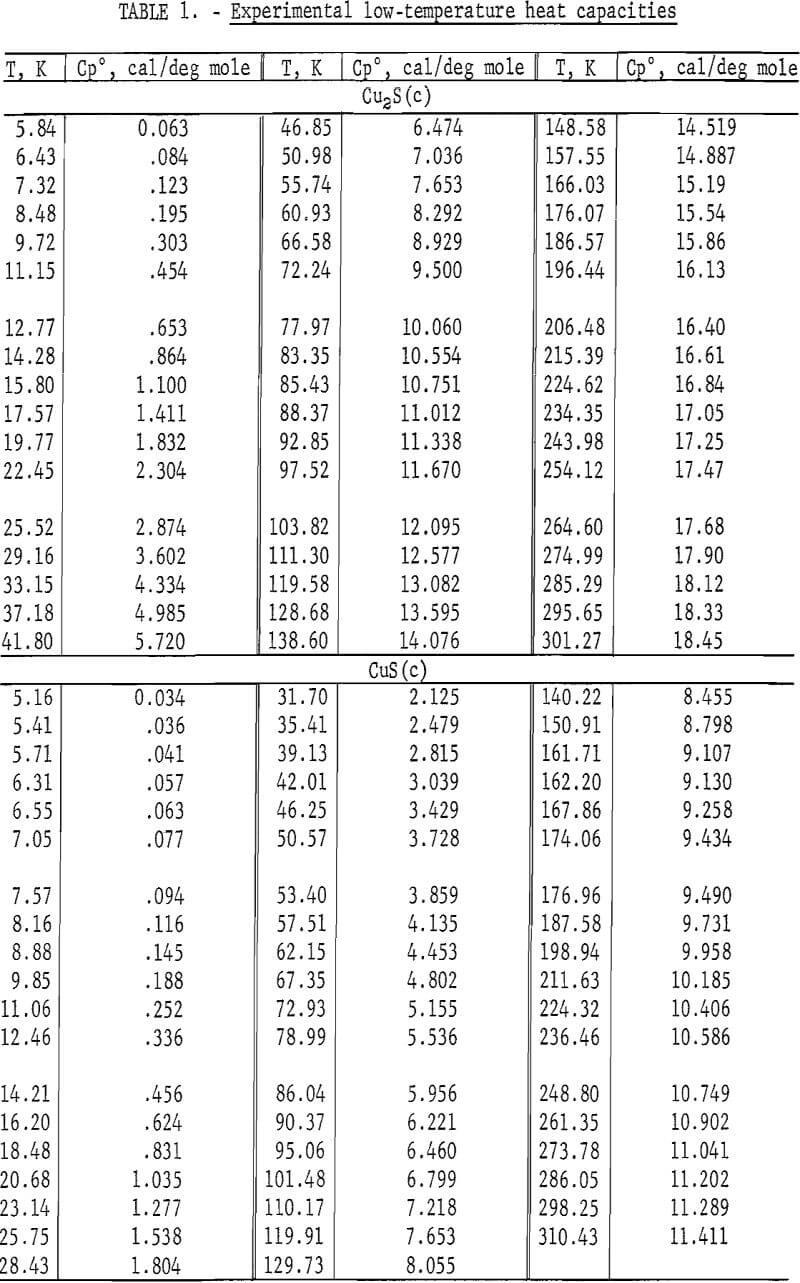
:max_bytes(150000):strip_icc()/GettyImages-154953454-6806780a2f0f4ec99daf580619b5aeef.jpg)