![PDF] Electronic Specific Heat of Iron Pnictides Based on Electron-Cooper Pair Interaction | Semantic Scholar PDF] Electronic Specific Heat of Iron Pnictides Based on Electron-Cooper Pair Interaction | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/36a34a8a61709b713d9e6e7e12f0b93504140c9c/8-Figure3-1.png)
PDF] Electronic Specific Heat of Iron Pnictides Based on Electron-Cooper Pair Interaction | Semantic Scholar

Composition and structure dependence of specific heat of disordered iron-palladium alloys - ScienceDirect
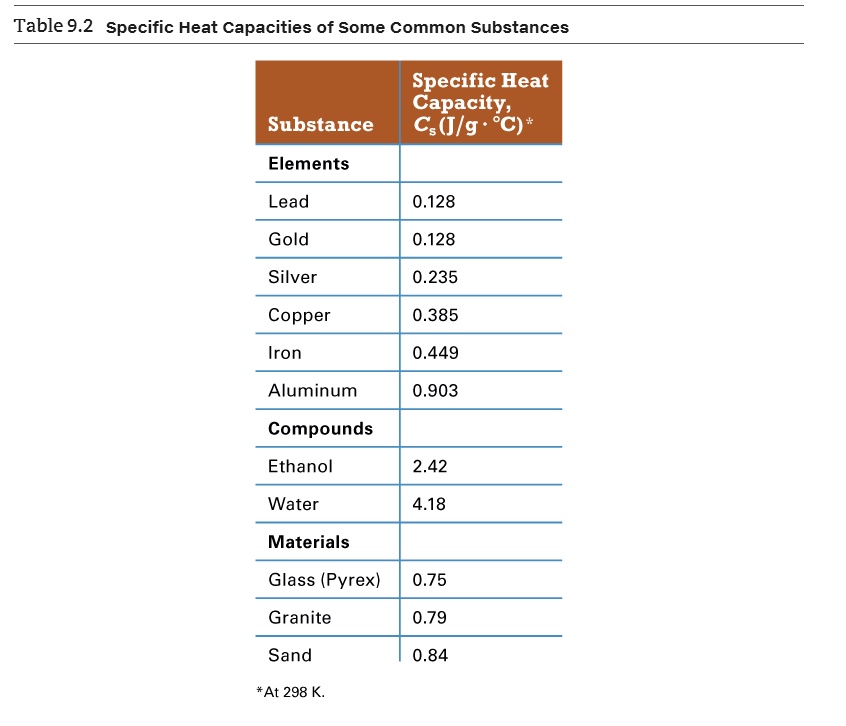
SOLVED: Table 9.2 Specific Heat Capacities of Some Common Substances Specific Heat Capacity; Cs(Jlg. *C) Substance Elements Lead 0.128 Gold 0.128 Silver 0.235 Copper 0.385 Iron 0.449 Aluminum 0.903 Compounds Ethanol 2.42

6pc Specific Heat Metal Cylinders Set - Copper, Lead, Brass, Zinc, Iron & Aluminum - Includes Wooden Storage Block - for Specific Heat, Specific Gravity & Density Experimentation - Eisco Labs: Amazon.com:
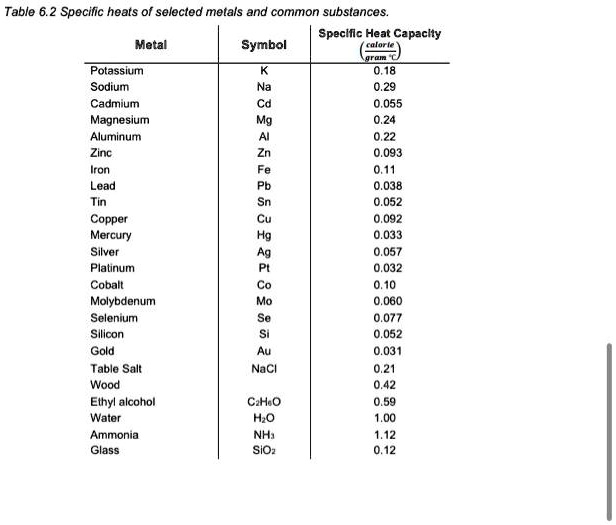
SOLVED: Table 6.2 Specific heats of selected metals and common substances: Speclllc Heat Capacly Metal Symbol Potassium Sodium Cadmium Magnesium Aluminum Zinc Iron Lead 0,18 055 093 0,11 0.038 052 092 033

Derived mean values of the specific heat of pure iron in comparison... | Download Scientific Diagram

Color online) Temperature-dependent specific heat capacities of (a)... | Download Scientific Diagram

A sample of iron absorbs 81.0 J of heat, upon which the temperature of the sample increases from 19.7 - Brainly.com
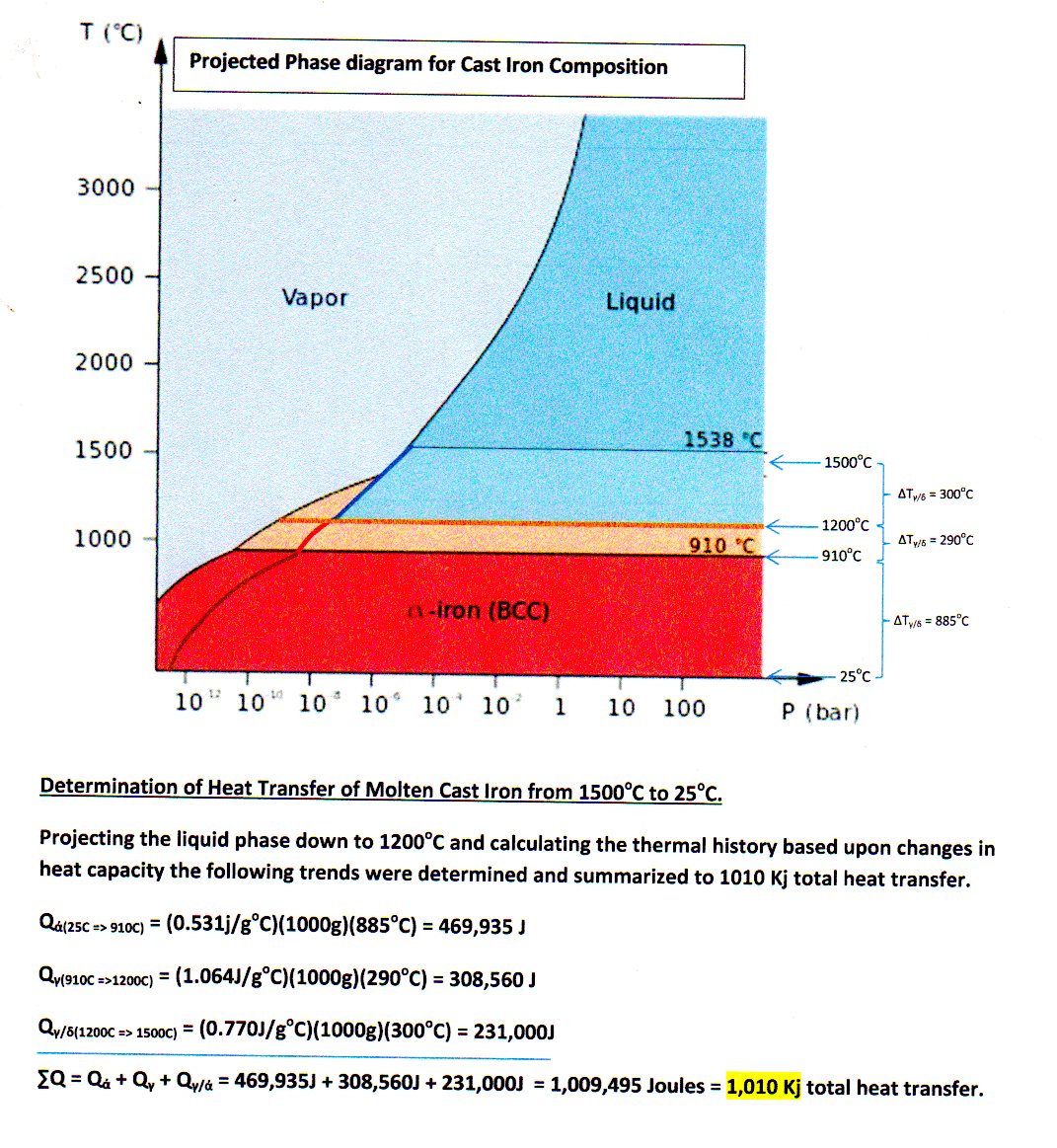
Molten iron is extremely hot, averaging about 1,500 C. The specific heat of iron is 0.46 J/gC. How much heat is released to the atmosphere when 1 kg molten iron cools to
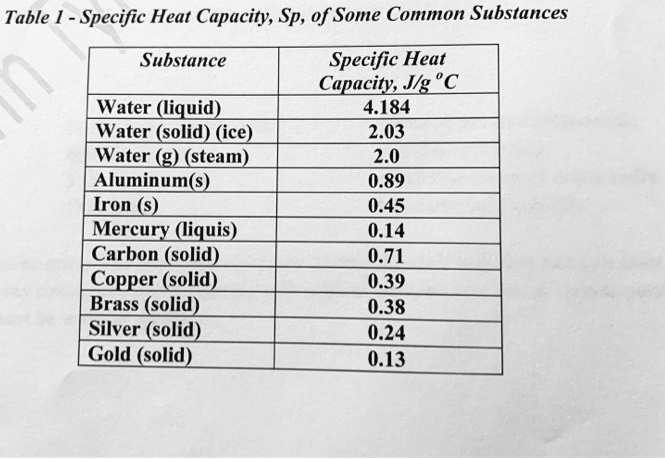

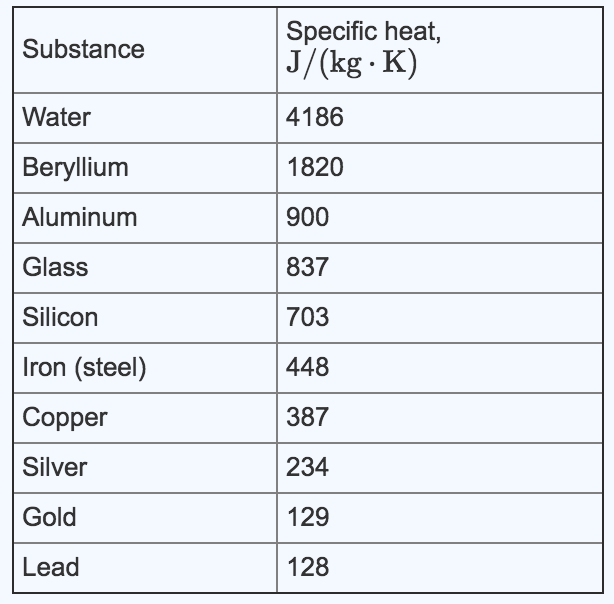


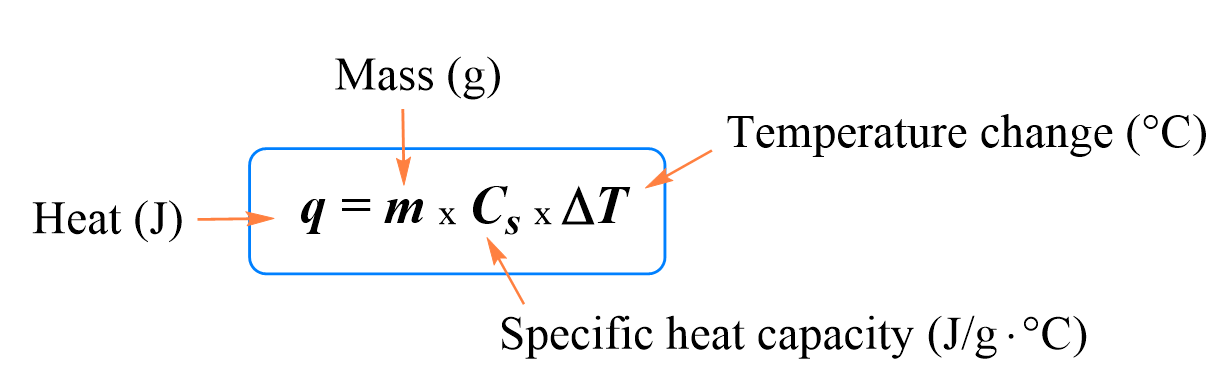



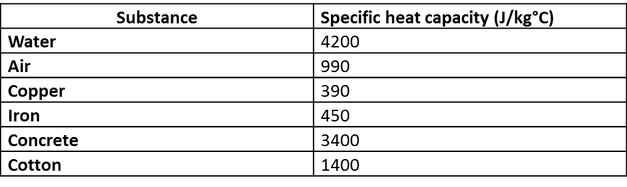

![Q. 3.37 If the same amount of heat is su... [FREE SOLUTION] | StudySmarter Q. 3.37 If the same amount of heat is su... [FREE SOLUTION] | StudySmarter](https://studysmarter-mediafiles.s3.amazonaws.com/media/textbook-exercise-images/image_fqdOuye.png?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA4OLDUDE42UZHAIET%2F20230619%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20230619T113149Z&X-Amz-Expires=90000&X-Amz-SignedHeaders=host&X-Amz-Signature=9addeb669c18772e2f62a5496f76a61c0c41bc11c2f7d3c5d66f3d6f112adfcc)
